INTRODUCTION
In modern society, a large number of people, regardless of gender or age, smoke cigarettes, and cigarettes have become a major area of social life as a favorite item.1 In the Republic of Korea, as of 2021, 16.4% of those over the age of 15 smoke cigarettes, which is similar to the OECD average.2 Smoking has become one of the top priorities of health promotion policies in that it damages the health of both smokers and non-smokers alike. There were 58,036 cases (50,942 males and 7094 females), with an associated mortality rate of 32.3% for males and 5.3% for females.3 The physiological effects of smoking on the respiratory system are well known, leading to substantial discussions about the clinical importance of assessing respiratory function in smokers.4,5
Breathing plays an essential role in basic life-support functions, stabilization of balance and posture, stress, and pain.6 Breathing patterns are determined by the part of the body where the most movement occurs during breathing,5 and there are differences in breathing patterns between smokers and non-smokers. Smokers require more ventilation for breathing than non-smokers during rest or exercise, so relatively more effort is required for ventilation.1 As a result, additional mobilization of the inspiratory and expiratory muscles is required, resulting in changes in the respiratory system.1 Therefore, smoking reduces cardiorespiratory function and causes respiratory diseases, which impairs ventilation and respiration functions and changes the mechanism of thoracic cage movement.7
According to previous study, the maximum oxygen intake of smokers is lower than that of non-smokers of the same age.4 Relatedly, smokers’ aerobic exercise capacity is reduced compared to that of non-smokers because of carbon monoxide in cigarette smoke, decreased ability of hemoglobin to supply oxygen to the blood, and insufficient oxygen supply to cells.4 In order to strengthen weakened lung capacity and increase aerobic exercise capacity, smokers can perform aerobic exercises, such as jogging, cycling, and running. In addition, smokers can purchase exercise equipment that strengthens respiratory muscles and perform exercises to improve the functioning of the diaphragm, which is used for abdominal breathing. Through aerobic and respiratory muscle strengthening exercises, smokers can normalize abnormal breathing patterns.
Differences in breathing patterns between smokers and non-smokers are evident during high-intensity exercises. In these exercises, airway resistance among chronic smokers has been shown to be more than twice as high as that of non-smokers. However, it has also been reported that airway resistance decreases in those who quit smoking 24 hours before exercising.8 In a previous study that compared lung function between smokers and non-smokers, smokers, in contrast to non-smokers, exhibited a decrease in vital capacity, forced expiratory volume in one second (FEV1), and maximum ventilation.4 Therefore, smokers require more respiratory volume than non-smokers when performing any exercise, which requires coordinated and organic mobilization of both the main and accessory respiratory muscles.9 In other words, as the amount of ventilation is insufficient only with abdominal breathing, such insufficiency can be resolved through thoracic breathing that mobilizes the sternocleidomastoid (SCM) and pectoralis minor, which are secondary thoracic respiratory muscles (accessory muscles of respiration).
Unlike abdominal respiration, which involves inhalation through the diaphragm and exhalation through the elasticity of the lungs, thoracic respiration uses the upper chest muscles, including the scalenus, to inhale.10 Therefore, thoracic breathing can limit abdominal breathing via the diaphragm due to hypertrophy and shortening of the SCM muscle, pectoralis minor muscle, and continuous tension of the abdominal muscles.10
When the SCM and pectoralis minor muscles, which are accessory muscles of respiration, are overused and hypertrophied, abdominal breathing capacity decreases and unnecessary thoracic breathing becomes excessive, which deforms the basic pattern of breathing and causes unnecessary changes. Despite these problems, studies that have analyzed the overuse of breathing accessory muscles or changes in lung capacity in smokers during aerobic exercise have thus far been insufficient. Therefore, the present study aimed to determine the extent to which subjects whose lung capacity was reduced due to smoking overuse their accessory muscles of respiration compared to non-smokers to compensate for the lack of lung capacity during stair-climbing exercises.
METHODS
This study recruited 10 males and 10 females who were not restricted from performing aerobic exercises, according to the following criteria: (1) adults over 20 years of age, (2) smokers who smoked 13 or more cigarettes per day, (3) smokers who have smoked for more than three years, (4) no history of physical or mental problems, (5) no difficulty breathing during exercise and (6) not currently taking medication.
Among the selected subjects, through individual interviews, those with a past history related to the cardiovascular system and respiratory system, those with current cardiopulmonary disease, those with cognitive function abnormalities, those with pain and discomfort in breathing during aerobic exercise. Those who did not agree with the procedure were excluded from the study. Subjects who met the study conditions and criteria were selected, the purpose and method of the study, and expected effects were explained, and the experiment was conducted after obtaining consent to participate in the study. The 20 subjects ultimately included in the study were divided into two groups: smokers (n=10) and non-smokers (n=10). The general characteristics of the subjects are shown in Table 1. This study was approved by the Baekseok University Human Studies Committee.
| Characteristics | Smokers group (n=10) | Non-smokers group (n=10) |
|---|---|---|
| Age (yrs) | 24.5±1.96 | 24.2±1.4 |
| Height (cm) | 170.9±8.03 | 177.2±4.64 |
| Weight (kg) | 69.9±15.26 | 73.4±11.53 |
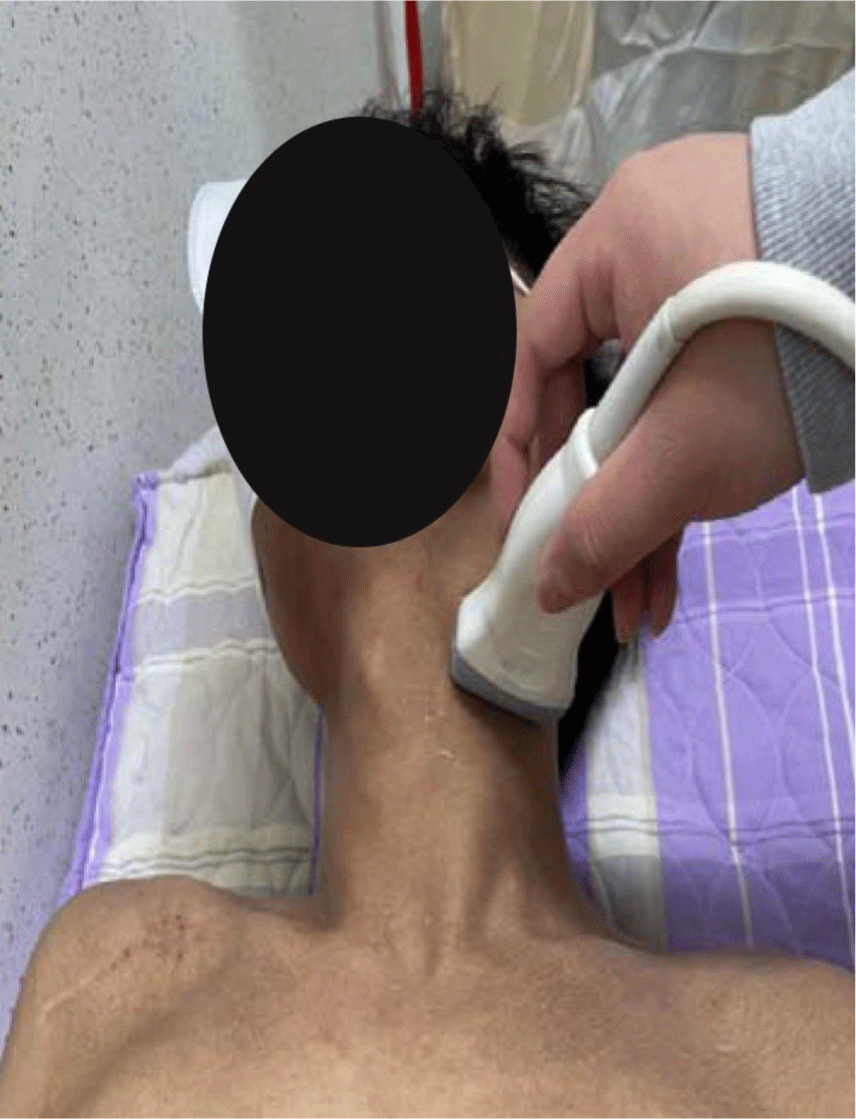
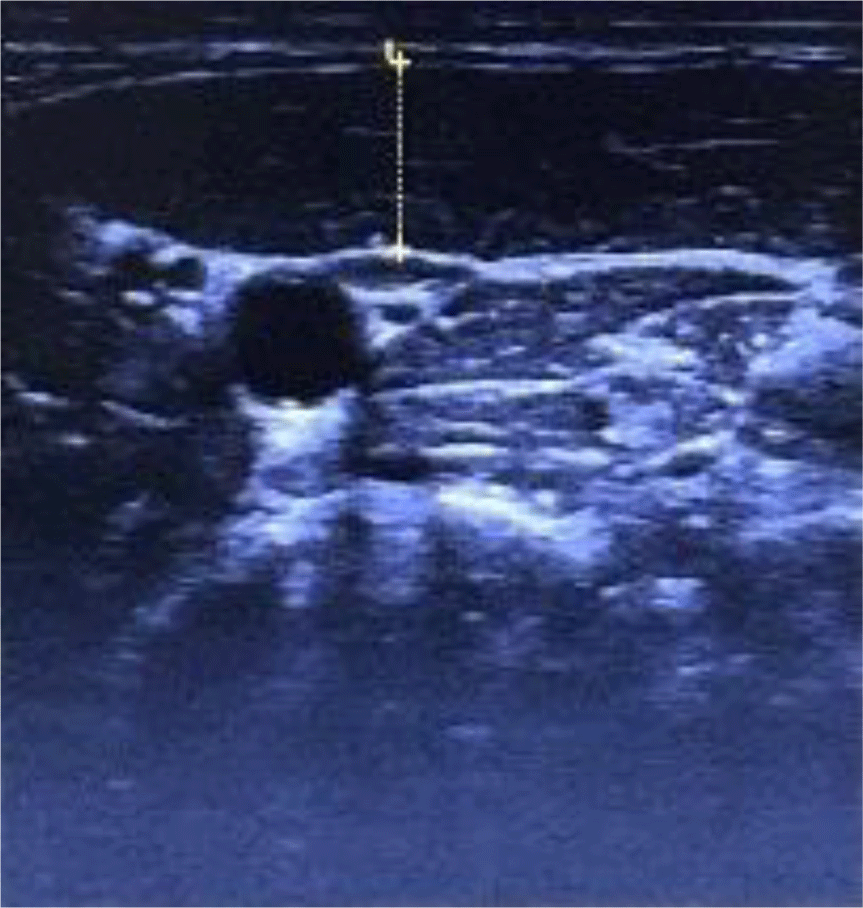
In this study, an ultrasonic device, LOGIQ P6 PRO (GE Inc., New Jersey, USA), was used to measure the thickness of the SCM muscle. For these measurements, a linear probe set to B-mode was employed. The subjects’ necks were rotated to the opposite side in a supine position, after which a semi-solid conducting gel for ultrasound measurement was spread across both SCM muscles.11 After palpating the fifth cervical vertebra (C5) according to Cagnie et al.,12 the SCM muscle was measured at this level. For C5 palpation, the measurer palpated the laryngeal prominence with the second finger and marked the tip of the third finger with a marker. This point was 1.5 cm below the occipital prominence and corresponded to C5. The center of the probe was placed at this point, with the probe perpendicular to the longitudinal axis of the subject’s neck. The thickness of the SCM muscle was measured on the lateral side of the carotid artery. The muscle visible at the top of the cross-sectional image of the neck is the SCM muscle.13 In order to minimize error, the average value, which was measured three times, was used as the data value (Figure 1 and 2).
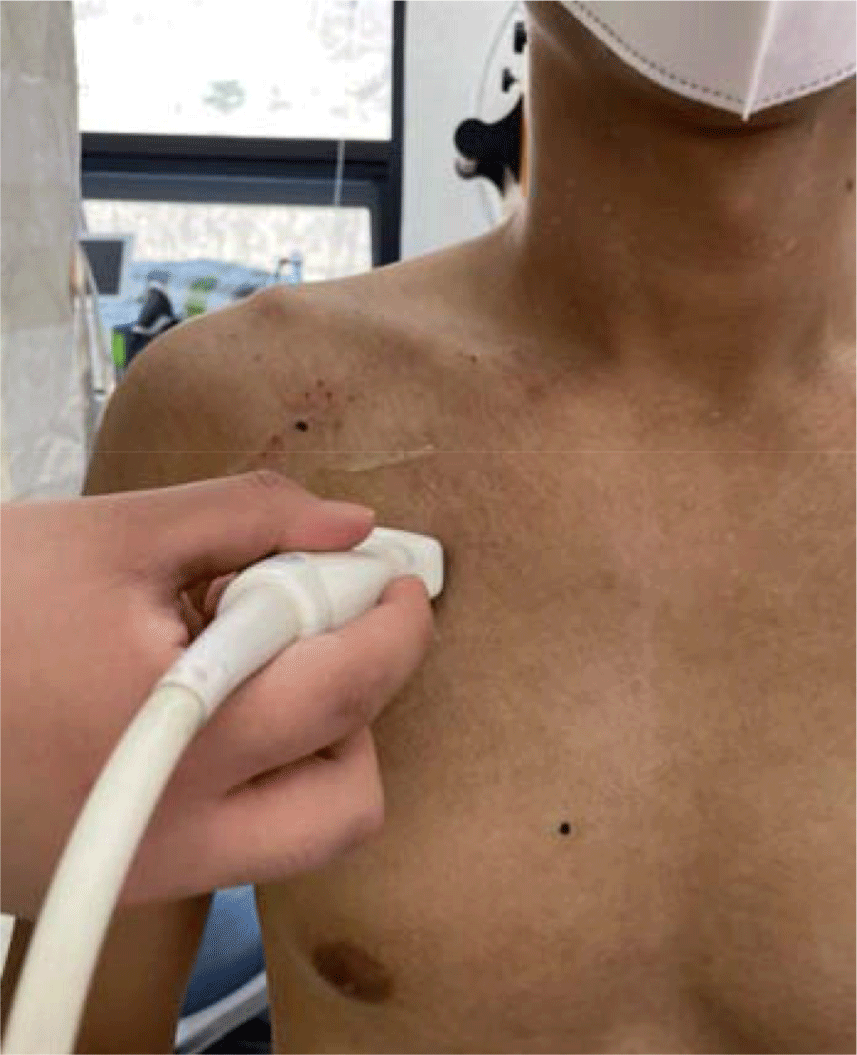
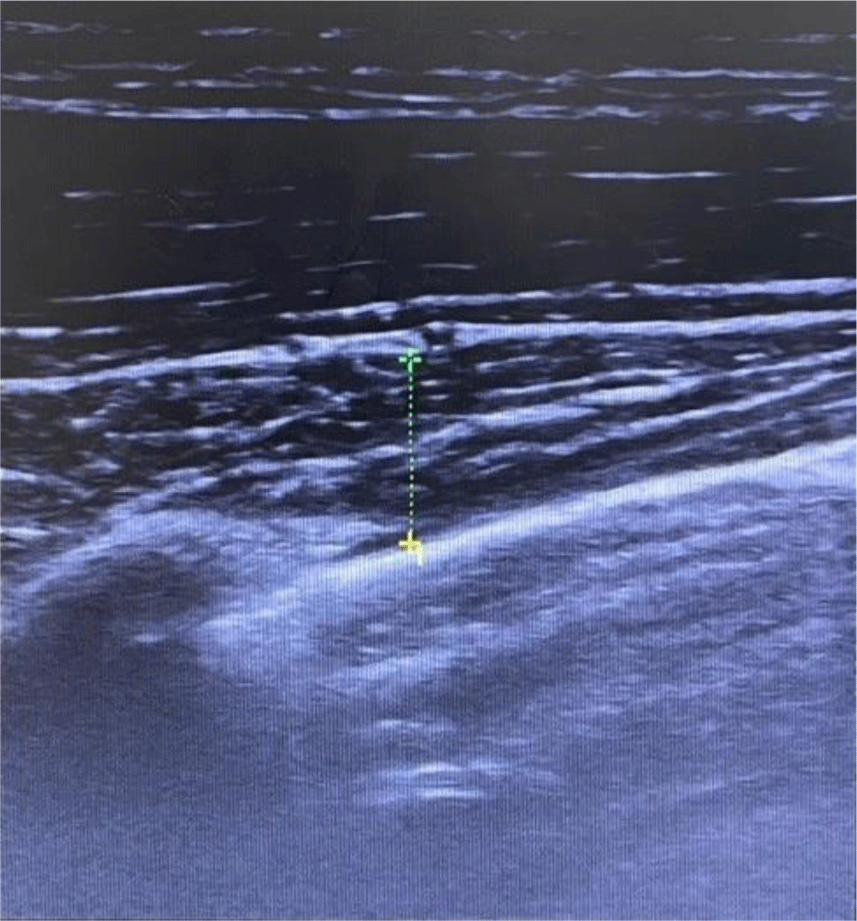
Measurement of the thickness of the pectoralis minor muscle was performed in the correct sitting position with the subjects’ hips and knees bent at 90° on a chair with a backrest and their hands placed beside their thighs. For the location of measurement, the coracoid process and the 4th rib were palpated in consideration of the tooth and the contact point, with these two points set as indicators. The probe was placed 1/3 of the point where the line connecting the beak process and the 4th rib reached. With the subject seated in a comfortable position, the probe was placed on the transverse axis of the pectoralis minor muscle and an image was obtained at the end of exhalation to minimize the effect of respiration and muscle contraction.14 The length of the pectoralis minor muscle was measured at the center of the ultrasound screen. In order to minimize error, the average value, which was measured three times, was used as the data value (Figure 3 and 4).
The respiratory function of the subjects in this study was assessed using spirometry (Pony Fx MIP/MEP, Cosmed Srl, Korea), which measured FVC and FEV1. FVC refers to the amount of air blown out after inhaling air as quickly and forcefully as possible.15 FEV1 is the volume of air exhaled in the first second when maximal inhalation occurs and exhalation occurs with effort, like forced lung capacity.8 For accurate spirometry, the subjects were provided with a sufficient explanation of the procedure.16 When in a supine position, the abdominal organs and muscles compress the diaphragm such that lung capacity is generally lower than when in a sitting position.17 Therefore, in order to accurately measure FVC and FEV1, the subjects sat in the correct sitting position on a chair, with their legs spread a shoulder-width apart and placed perpendicular to the floor, and their back and shoulders straight.8 In order to reduce muscle fatigue, a 1-minute break was provided between each of the three measurements.
The subjects sat for 15 minutes before the start of the experiment to ensure they were sufficiently rested. Aerobic exercises consisted of a warm-up exercise, ascending and descending stairs, and a cool-down exercise. The warm-up and cool-down exercises were performed for 5 minutes each, and the stair-climbing exercise was performed for 20 minutes. In order to maintain a constant speed when ascending and descending the stairs, each step was taken according to a metronome set to 120 bpm. For the warm-up and cool-down exercises, national gymnastics was employed to relieve tension in the legs.
In this study, SPSS 18.0 for Windows was used for the processing of statistical data. Descriptive statistics were used to describe the general characteristics of the subjects. A paired t-test was used to compare differences in the thickness of the accessory respiratory muscles and lung capacity before and after ascending and descending the stairs in each group, and the difference between the thickness of the breathing accessory muscle and the lung capacity before/after were compared between the groups. For this, an independent t-test was used. The level of statistical significance was set at 0.05.
RESULTS
The mean difference in the thickness of the SCM muscle before and after ascending and descending the stairs among members of the smoker group was 0.11±0.62 cm, whereas that of members of the non-smoker group was 0.05±0.17 cm. After ascending and descending the stairs, the thickness of the sternocleidomastoid muscle increased significantly (p<0.05) (Table 2, 3, and 4).
The mean difference in the thickness of the pectoralis minor muscle before and after ascending and descending the stairs among members of the smoker group was 0.22± 0.09 cm, whereas that of members of the non-smoker group was 0.08±0.04 cm. Thus, the thickness of the pectoralis minor muscle increased significantly more among members of the smoker group after ascending and descending the stairs compared to members of the non-smoker group (p< 0.05) (Table 2, 3, and 4).
The mean difference in FVC before and after ascending and descending the stairs among members of the smoker group was 0.06±0.11 L, whereas that of members of the non-smoker group was 0.02±0.16 L. Therefore, no significant difference was found in FVC between members of the smoker group and members of the non-smoker group (p>0.05). The mean difference in FEV1 before and after ascending and descending the stairs among members of the smoker group was 0.34±0.49 L, whereas that of members of the non-smoker group was 0.10±0.33 L, which was significant between the smoker group and the non-smoker group. There was no difference (p>0.05) (Table 2, 3, and 4).
DISCUSSION
In this study, an experiment was conducted by dividing smokers and non-smokers into two groups to determine whether differences existed between them in terms of the thickness of the SCM and pectoralis minor muscles, FVC, and FEV1 during stair-climbing exercise. The results showed that after ascending and descending the stairs, the thickness of the SCM muscle and pectoralis minor muscle increased significantly among members of the smoker group than before the exercise. In contrast, no significant differences in lung capacity were found between or among members of the smoker and non-smoker groups before and after performing the exercise.
According to a previous study, the airway resistance of chronic smokers is more than twice as high as that of non-smokers during high-intensity exercise,8 and it has been reported that organic movements of the abdominal respiratory muscles and breathing accessory muscles are required to increase respiratory volume in smokers.9 Similarly, in the present study, it was found that smokers also mobilized the SCM muscle and pectoralis minor muscle to compensate for insufficient respiratory volume with thoracic breathing during the stair-climbing exercise.
The SCM not only maintains the posture of the neck but is also one of the most important muscles in breathing. When the SCM muscle is frequently used and hypertrophied, causing fatigue and pain in this muscle, breathing dysfunction may occur.18 In addition, due to hypertrophy and shortening of the SCM muscle, a forward head posture may occur, which can cause pain in the neck and shoulder area, resulting in an imbalance in the strength and endurance of the muscles around the neck and shoulder.19,20 This in turn leads to a slouched posture of the spine, restricts the mobility of the thoracic cage, and interferes with the movement of the diaphragm, which can culminate in general breathing problems, such as decreased lung capacity.21
Since the pectoralis minor muscle attaches to the coracoid process of the scapula, a shortening of this muscle causes the scapula to tilt forward and deviate from normal shoulder alignment.22,23 Also, in people with rounded shoulders, the pectoralis minor muscle is shortened and, as the degree of shortening increases, scapular misalignment increases.24 When the pectoralis minor muscle is enlarged or shortened, it compresses the nerves and blood vessels passing under the muscle, causing thoracic outlet syndrome.25
In other words, when smokers repeatedly and frequently mobilize the SCM muscle and pectoralis minor muscle to compensate for an insufficient amount of ventilation when ascending and descending stairs, as shown in the results of this study, shortening or hypertrophy of the two muscles may occur, which, as mentioned above, is the cause of various musculoskeletal disorders.
Roh observed an increase in lung capacity after treadmill walking for 60 minutes per day, 3 times per week, for 12 weeks, while another study demonstrated an increase in FEV1 during long-term treadmill walking for eight weeks.26,27 However, in the present study, lung capacity was measured after a single stair-climbing exercise rather than continuously during several stair-climbing exercises. Also, since all of the subjects were healthy young adults in their 20s, there may have been no significant difference in lung capacity between smokers and non-smokers.
Aerobic exercise is essential for improving cardiorespiratory function.28 However, when smokers seek to improve lung capacity through the same aerobic exercises used by non-smokers, they have greater difficulty doing so effectively because they must mobilize thoracic breathing, as mentioned above, and musculoskeletal problems may occur. Excessive thoracic breathing using the breathing accessory muscles causes tension in these muscles, such as the SCM muscle and pectoralis minor muscle, which can lead to postural imbalance,29 continuous abdominal muscle tension, and difficulty with abdominal breathing using the diaphragm. This can in turn induce even more thoracic breathing. Continuous thoracic breathing breaks the basic breathing pattern, resulting in a respiratory imbalance. This imbalance can culminate in a vicious cycle due to increased use of the breathing accessory muscles to maintain breathing.30
Therefore, smokers should carefully select and perform aerobic exercises by limiting thoracic breathing and promoting abdominal breathing as well as by performing lower-intensity exercises than those used by non-smokers. In addition, it is recommended that smokers, when performing aerobic exercises, pay greater attention to the fact that breathing accessory muscles can become hypertrophic due to incorrect breathing patterns.
This study had some limitations that should be addressed in future studies. First, the subjects were mostly limited to adults in their 20s. However, chronic diseases caused by smoking have been found in 40.3% of those aged 40 to 64 and 75.5% of those aged 65 or older, compared to 11.7% of those in their 20s and 30s.31 Second, it was not possible to completely exclude factors affecting respiratory function because we could not control for aspects of the subjects’ daily life. Considering these points, future studies should seek to effectively control for external factors that can affect respiratory function, such as temperature and particulates, and should include subjects aged 40 or older, who are more prone to diseases caused by smoking.
CONCLUSIONS
Our results showed that the thickness of the SCM muscle and pectoralis minor muscle increased significantly among members of the smoking group compared to those in the non-smoking group after the stair-climbing exercise. Increased use of the breathing accessory muscles during aerobic exercise can negatively affect normal breathing patterns, reduce ventilation, and cause various musculoskeletal disorders due to misalignment of the neck and shoulders. Therefore, if the SCM muscle and pectoralis minor muscle are enlarged or shortened due to excessive use of the breathing accessory muscles during aerobic exercise, musculoskeletal disorders, such as rounded shoulder posture and thoracic outlet syndrome, may occur. Therefore, smokers should carefully select and perform aerobic exercises, such as by gradually improving lung capacity by exercising at a lower intensity than non-smokers.